Stem Cell Supernatant Infusion
Ideal for the following concerns
Types of Stem Cell Supernatant and Their Characteristics
(1) Fat-derived stem cell supernatant
(2) Umbilical cord-derived stem cell supernatant
(3) Dental pulp-derived stem cell supernatant
Comparison Table of Growth Factors in Stem Cell Supernatant
Procedure Flow of Stem Cell Supernatant
Recommended Infusion Schedule for Supernatant Based on Symptoms
Precautions for Stem Cell Supernatant Infusion Treatment
Price List
Stem Cell Supernatant Infusion
Stem cell culture supernatant infusion therapy is a treatment where the liquid containing growth factors and cytokines secreted during the process of culturing human-derived stem cells for stem cell therapy is administered intravenously.
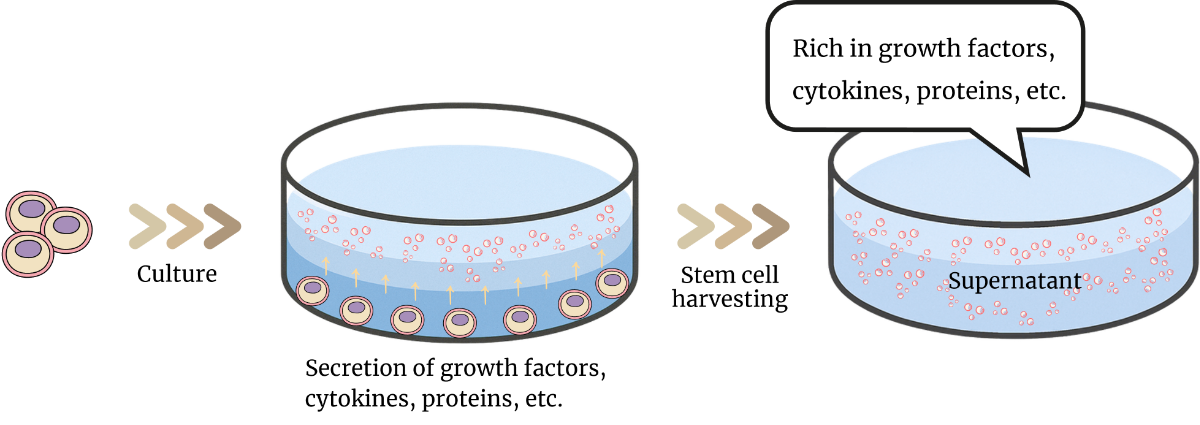
Sources of these stem cells include dental pulp, umbilical cord, bone marrow, and fat, but at our clinic, we use human umbilical cord-derived, human dental pulp-derived, and human fat-derived stem cell culture supernatant. The key feature of this treatment is that it uses allogeneic (donor-derived) stem cells, which means there is no need for cell harvesting, making it easier for patients to undergo the treatment. Tokyo Relife Clinic also offers autologous stem cell culture and infusion therapy so patients who prefer can choose to use autologous stem cell culture supernatant.
Using allogeneic supernatant eliminates the need for fat extraction and cell culture, making the process more convenient. The growth factors and other substances in the supernatant can help promote tissue repair, reduce inflammation, and regulate the immune system.
Ideal for the following concerns:
- Wrinkles
- Sagging
- Improved skin elasticity
- Improvement of spots and dullness
- Sleep disorders
- Post-COVID syndrome
- Fatigue recovery
- Immune system enhancement
- Rejuvenation of internal organ functions
- Post-stroke sequelae
- Alzheimer's disease
- Improvement of neurological disorders
- Sports injuries
- Muscle fatigue
- Atopic dermatitis Improvement of allergy constitution
- Diabetes
Types of Stem Cell Supernatant and Their Characteristics
(1) Fat-derived Stem Cell Supernatant
✔ Characteristics: Rich in growth factors, excellent for aesthetic purposes and skin regeneration
✔ Indications: Skin rejuvenation, anti-aging, wrinkle and sagging improvement
For aesthetic purposes, stem cell supernatant treatment can also be performed through Water-light injection using the supernatant.
(2) Umbilical Cord-derived Stem Cell Supernatant
✔ Characteristics: Strong anti-inflammatory and immune-modulating effects, suitable for tissue repair
✔ Indications: Autoimmune diseases, neurological disorders, chronic fatigue, joint repair
For joint treatment, supernatant infusion is recommended.
For post-COVID syndrome, nebulizer inhalation therapy is recommended.
(3) Dental Pulp-derived Stem Cell Supernatant
✔ Characteristics: Contains many neuroregenerative factors, excellent for repairing nerve cells
✔ Indications: Post-stroke sequelae, Alzheimer's disease, improvement of neurological disorders
For post-stroke sequelae, Alzheimer's disease, and psychiatric disorders, dental pulp nasal administration is recommended.
Our clinic handles all three types of supernatant, but the ones offered for infusion therapy are fat-derived stem cell supernatant and umbilical cord-derived stem cell supernatant.
Comparison Table of Growth Factors in Stem Cell Supernatant
Growth Factor | Fat-derived | Umbilical Cord-derived | Dental Pulp-derived | Main Functions |
|---|---|---|---|---|
VEGF (Vascular Endothelial Growth Factor) | ◎ | ○ | ◎ | Promotes angiogenesis, improves blood flow |
KGF (Keratinocyte Growth Factor) | ◎ | △ | ○ | Promotes skin and hair growth, wound healing |
HGF (Hepatocyte Growth Factor) | ◎ | ○ | △ | Liver function recovery, tissue regeneration, anti-inflammatory effects |
PDGF (Platelet-Derived Growth Factor) | ◎ | ○ | ○ | Tissue repair, wound healing, fibroblast activation |
bFGF (Basic Fibroblast Growth Factor) | ○ | ◎ | ◎ | Promotes collagen and elastin production, improves wrinkles |
TGF-β (Transforming Growth Factor β) | ○ | ◎ | ○ | Immune regulation, anti-inflammatory effects, wound healing |
IGF-1 (Insulin-like Growth Factor 1) | ○ | ◎ | ○ | Promotes muscle, bone, and skin growth, prevents aging |
SCF (Stem Cell Factor) | △ | ◎ | ○ | Regulates stem cell self-replication and differentiation |
GDNF (Glial Cell-Derived Neurotrophic Factor) | ○ | ○ | ◎ | Protects dopaminergic neurons, promotes motor neuron survival |
BDNF (Brain-Derived Neurotrophic Factor) | ○ | ○ | ◎ | Promotes neuronal survival, synaptic plasticity, learning, and memory |
NGF (Nerve Growth Factor) | △ | ○ | ◎ | Promotes survival, differentiation, and axon growth of peripheral nerves |
※This comparison table is for reference only. In reality, there may be significant variations due to differences in collection methods, culturing environments, and measurement techniques.
Procedure Flow
- Consultation & Examination: Confirm treatment goals and check physical condition
- Infusion Preparation: Mix stem cell supernatant with saline
- Infusion: Approximately 30 to 60 minutes
- Post-treatment Rest: Rest for about 10 to 15 minutes
- Aftercare & Follow-up Observation: Check condition 1 to 2 weeks after treatment
Recommended Infusion Schedule for Supernatant Based on Symptoms
Treatment Purpose | Initial Infusion | Maintenance Therapy |
|---|---|---|
Skin Rejuvenation & Firmness Improvement | The first 3 to 5 treatments are given every 3 to 4 weeks. | Once every 1 to 3 months |
Osteoarthritis & Arthritis | The first three treatments are given every 1 month. | Once every 6 months |
Immunity Boost & Fatigue Recovery | The first three treatments are given every 1 month. | Once every 3 to 6 months |
Neurological Disorders (Dementia, Post-Stroke Sequelae) | The first 3 to 5 treatments are given every 1 to 2 months. | Once every 3 to 6 months |
Precautions for Stem Cell Supernatant Infusion Treatment
Precautions Before Treatment
- Since stem cell culture supernatant is a human-derived product, current testing methods cannot completely rule out the possibility of unknown viral infections. Therefore, individuals who have received even one infusion of stem cell culture supernatant will no longer be eligible for blood donation.
- The stem cell culture supernatant used in our clinic is rigorously tested and confirmed to be free of general bacteria, fungi, mycoplasma, and the following viruses: Hepatitis B Virus (HBV), Hepatitis C Virus (HCV), Human Immunodeficiency Virus (HIV), Adult T-cell Leukemia Virus (HTLV), Parvovirus B19, Epstein-Barr Virus (EBV), Cytomegalovirus, Herpes Simplex Virus, Varicella-Zoster Virus, Human Herpesvirus (HHV-6/7/8), Adenovirus, JC Virus, Polyomavirus, Human Papillomavirus, and Bovine Albumin. In addition, products will undergo tests for organic substances and viruses before shipment to ensure safety. However, there is still a theoretical possibility of transmitting infections caused by currently unidentified pathogens.
Side Effects and Downtime
- Swelling & Redness: Swelling or redness may occur at the injection site, but it typically improves within a few days.
- Needle Marks: Temporary redness may remain at the needle insertion site immediately after treatment, but it usually fades within a few days.
- Bruising: Bruising may occur at the infusion site, but it generally resolves within a few days.
- Allergic Reactions: In rare cases, redness, itching, or eczema-like symptoms may appear.
※ The occurrence of side effects varies from person to person, but please proceed with the treatment with an understanding of the potential risks.
Precautions After Treatment
- To avoid causing bruising, please do not rub or strongly press the insertion site.
People Who Should Not Receive Stem Cell Supernatant Treatment
- Individuals under 18 years old
- Pregnant, breastfeeding, or those who may be pregnant
- People with malignant tumors (※1)
- People with viral infections
- Those allergic to antibiotics (penicillin, aminoglycosides, macrolides)
- Those who have received a vaccine within the past month or are scheduled to receive one within the next two weeks
- People with severe diabetes, liver disease, kidney disease, or heart failure
※ Eligibility is determined by the doctor's diagnosis. You may be unable to receive treatment even if the above conditions do not apply to you.
※1 Individuals with active malignant tumors or those under observation after malignant tumor treatment may not be able to receive treatment.