Exosome Infusion Treatment
Exosome infusion treatment represents a cutting-edge approach in regenerative medicine that has gained significant attention in recent years.
In this therapy, only exosomes (extracellular vesicles) secreted by stem cells are carefully extracted and purified from the culture supernatant of stem cells derived from sources such as fat tissue or bone marrow, and then administered through intravenous infusion.
Exosomes carry miRNA (microRNA), which efficiently delivers repair and recovery signals to cells throughout the body.
Their effects include cellular rejuvenation, improvement of vascular endothelial function, anti-inflammatory effects, and neuroprotective support, helping maintain tissue health and promote functional recovery.
For these reasons, exosome IV therapy is considered to have broad potential—not only for addressing age-related changes, but also for supporting a wide range of health conditions.
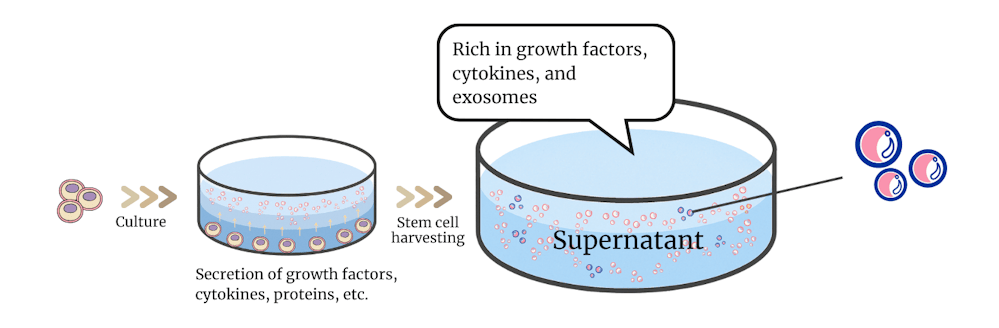
What are Exosomes?
In recent years, exosomes have garnered significant attention in the fields of cosmetics and aesthetic medicine.
Exosomes are actually extracellular vesicles released by almost all cells. They are extremely small, with a diameter of about 30–150 nm (1 nm = one billionth of a meter), and are nano-sized vesicles surrounded by a double lipid membrane.
In the past, exosomes were thought to be mere “cellular waste” produced during metabolism.
However, current research has shown that they play a crucial role in cell-to-cell communication and can directly regulate cellular functions.
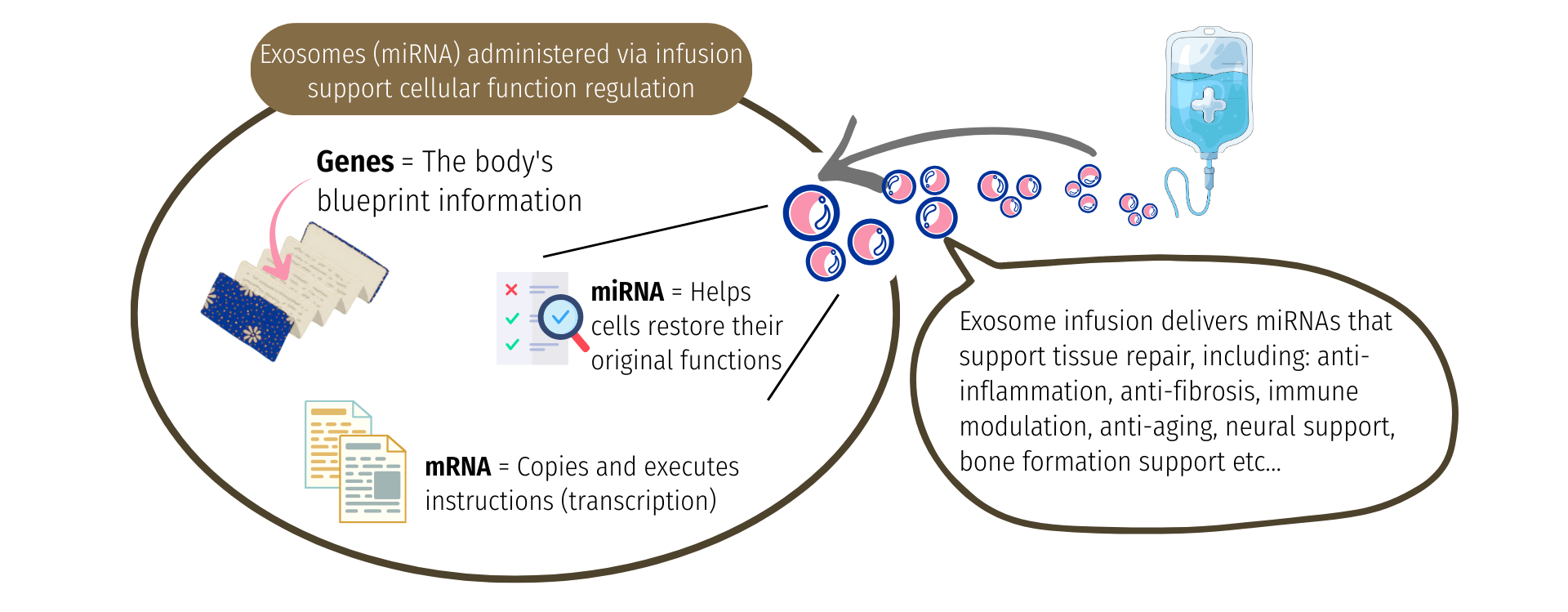
Because exosomes are protected by a double lipid membrane, their internal contents are shielded from breakdown by enzymes in the body. In addition, specific membrane proteins and sugar chains on their surface act as recognition markers, allowing target cells to accurately identify and efficiently take in the exosomes they need.
At Tokyo Relife Regenerative Medicine Clinic, in addition to exosome IV therapy, we also offer a variety of exosome-based treatments and care options, including:
- Exosome inhalation therapy (for lung function support)
- Home-use exosome inhalation (for lung health)
- Exosome nasal drops (for brain function support)
Please note that the most appropriate treatment method will be determined by the physician after consultation.
Target Areas for Improvement
- Wrinkles and skin sagging
- Skin firmness and elasticity
- Dark spots and dull skin tone
- Hair loss or thinning hair
- Chronic fatigue
- Support for recovery after injury or surgery
- Vascular aging
- Chronic joint conditions (or Chronic joint pain)
- Impaired liver function (or Liver health support)
- Prevention of cognitive decline
- Prevention of lifestyle-related diseases
- Male vitality and functional maintenance
- Declining memory
Why Exosome IV Therapy is Popular
Stem cell therapy—whether given by IV infusion or local injection—requires a minor surgical procedure to collect fat tissue, isolate stem cells, and culture them.
Exosome IV therapy, on the other hand, uses only the substances secreted by stem cells. Because no tissue collection or surgery is needed, patients can experience effects similar to stem cell IV therapy in a much simpler and less invasive way.
This ease and convenience are the main reasons why exosome IV therapy has become so popular.
Key Characteristics of Tokyo Relife Clinic's Exosomes
The exosomes used at Tokyo Relife Clinic are derived from dental pulp tissue and were developed with the involvement of Professor Takahiro Ochiai, who is widely regarded as a pioneer in exosome research.※1
※1 Source: Tokyo Medical University research report, June 2024
Item | Exosomes Used atTokyo Relife Clinic |
|---|---|
Source Cells | Dental pulp stem cells from healthy Japanese donors (ages 5–19) |
Cell Age | Genetically younger cells with strong proliferative capacity |
Exosome Quality | The right balance of biologically active signals needed for therapeutic effects |
Active Components | Each 1 mL contains approx. 5 billion exosomes, with a balanced composition of miR-24, miR-124, miR-133b, miR-26a, miR-125b, miR-181, and others |
Quality Control | Batch-by-batch testing to ensure consistency and minimize variability |

1.Features of Dental Pulp–Derived Exosomes
Because these exosomes are derived from baby teeth, the donor cells retain the inherent juvenility and high differentiation potential characteristic of human development.
Compared with cells from adults, they are in a low methylation state, which is associated with higher cellular activity.
These exosomes are also rich in miRNA related to nerve regeneration (such as miR-124, miR-133b, miR-26a, and miR-125b).
For this reason, they show potential for further research and application in areas such as the central nervous system, cognitive function, and difficult-to-treat neurological conditions.
2.Rich and Well-Balanced miRNA Profile
Dental pulp–derived exosomes are generally known to be dominated by miRNA related to neuroprotection, tissue repair, and cell differentiation, while containing relatively fewer miRNA related to anti-inflammatory or immune regulation.
However, miRNA profiling of the exosomes used here shows a more balanced composition. In addition to neuroprotective miRNA, they also contain immunosuppressive and anti-inflammatory miRNA (such as miR-21 and miR-146a), as well as angiogenesis-related miRNA (such as miR}-21) that support blood vessel formation, all configured in a well-balanced proportion.
Because of this balanced miRNA profile, these exosomes are not only suitable for neurological applications, which are typical for dental pulp–derived exosomes, but may also help regulate the immune system and overall metabolic balance.

3.Quality Evaluation and Control
In general, maintaining consistent quality in exosome products can be challenging, as their internal components may vary significantly between production batches. This is especially true when adult donors are used, since individual cell conditions can differ greatly, leading to variability in the final product.
The dental pulp–derived exosomes used atTokyo Relife Clinic are all obtained from baby teeth donated by healthy Japanese donors. Because baby teeth are still in a developmental stage, the cells are younger and more stable, resulting in less individual variation.
In addition, we apply strict quality control measures, including:
- Nanoparticle Tracking Analysis (NTA) to ensure consistency in particle size and concentration
- miRNA profiling (sequencing analysis) to confirm the presence and balance of specific functional miRNA
Through this multi-layered quality evaluation process, we do not focus only on the number of exosome particles. Instead, our key standard is whether each batch contains the right balance of biologically active signals needed for therapeutic effects.
Batches with large fluctuations in composition are excluded, clearly distinguishing our exosomes from products that emphasize particle count alone.
4.Liquid Cryopreservation (Non-Freeze-Dried) Method
Advantages of Liquid Cryopreservation | Risks of Freeze-Drying |
|---|---|
Preserves structural integrity | During freeze-drying, strong stress is applied to the exosome's double lipid membrane, increasing the risk of damage to key functional components (such as miRNA and proteins). |
Prevents loss of function | During reconstitution (rehydration) after powder formation, exosomes may aggregate, leading to reduced activity and effectiveness. |
No need for protective additives | To enable powderization, freeze-drying typically requires the addition of cryoprotectants (additives). |
Maintains maximum biological activity | Based on existing data, liquid cryopreservation is more effective in preserving the high biological activity required for clinical outcomes. |
Note: When administering exosomes by IV infusion, the double lipid membrane structure must remain intact, and preparation requires approximately one day to complete before treatment. For this reason, the reservation cutoff time for exosome IV therapy is 5:00 PM on the previous day. Please be aware of this in advance.
Treatment Process
- Consultation & Medical Assessment
The physician will review your current health condition and treatment goals to determine whether the therapy is suitable and to decide the appropriate dosage. Your current physical condition and concerns will be discussed, along with an explanation of the recommended dosage and treatment frequency. - IV Administration
Exosomes are administered slowly through an intravenous infusion. (Approximate duration: 30–60 minutes) - Post-Treatment Rest
After the infusion is completed, you will rest for about 10 minutes while your condition is monitored. - Aftercare
You may resume your normal daily activities after treatment. With regular treatments, more optimal results can be expected.
Sample Treatment Plans
Indication / Purpose | Dosage | Recommended Frequency | Expected Effect |
|---|---|---|---|
Prevention of lifestyle-related diseases | 30ml | Twice per month (initial phase) | Supports joint health, reduces joint inflammation, relieves mild pain |
Nervous system support | 30ml | Every 1–2 months | Supports nerve cell repair, relieves neurological symptoms, supports cognitive function |
Cognitive decline prevention / Memory support | 10~20ml | Every 3–6 months | Helps maintain memory and preserve brain function |
Anti-aging / General health maintenance | 5~10ml | Every 3–6 months | Systemic anti-inflammatory effects, improvement of chronic fatigue |
※The final dosage and treatment frequency will be determined by the physician based on consultation and examination results.
PRICE
Exosome Therapy (IV Drip)
Exosome IV Pre- and Post-Treatment Precautions
- If mild dehydration is present, intravenous access may be more difficult. Please hydrate regularly starting the day before your treatment. On the day of treatment, we ask that you drink approximately 500 mL of water about one hour before IV insertion.
- According to guidelines from the Ministry of Health, Labour and Welfare, this product is derived from human sources, and with current testing methods, the risk of unknown or as-yet-undetectable viral infections cannot be completely ruled out.
For this reason, once you have received exosome therapy, you will no longer be eligible to donate blood or organs in Japan. - Serious adverse events are rare; however, mild reactions such as fever, fatigue, or discomfort at the IV site may occur.
- You may resume normal daily activities immediately after exosome IV therapy.
However, please avoid strenuous exercise, alcohol consumption, and smoking on the day of treatment.
Who Is Not Eligible for Exosome IV Therapy
The following individuals are not suitable for exosome IV therapy:
- Under 18 years of age
- Pregnant or breastfeeding women, or those who may be pregnant
- Patients with malignant tumors*
- Those with an active viral infection
- Individuals who have received a vaccination within the past month, or plan to receive one within the next two weeks
- Patients with severe autoimmune diseases (such as systemic lupus erythematosus or rheumatoid arthritis)
- Patients with severe liver or kidney disease
- Individuals with a history of allergic reactions to components used during the cell culture process
※Other conditions deemed unsuitable by the physician
※※Patients with active malignancy or those still under follow-up after cancer treatment may not be eligible for this therapy
Note: Eligibility will be determined by the physician after consultation.
Frequently Asked Questions (FAQ)
Q:How many exosome infusions do I need?
A:The number of treatments varies depending on individual health conditions and treatment goals. Most patients choose to undergo multiple administrations. The specific number of sessions and intervals will be determined by your physician during consultation.
Q:What effects can I expect?
A:Exosome therapy is generally understood to support inflammatory regulation and tissue repair.
Q:How long do the effects last?
A:Depending on the dosage administered and individual differences, effects are typically believed to last approximately 3 months to 1 year.
Q:Are there any side effects or risks?
A:Serious adverse reactions are rare. However, temporary symptoms such as mild fever, fatigue, or discomfort at the infusion site may occur.
Q:How long does an exosome infusion take?
A:The procedure typically takes about 30 minutes. Depending on vascular conditions, it may take up to approximately 1 hour.
Q:Can I resume normal activities on the day of treatment?
A:Daily activities are generally not affected. However, please avoid strenuous exercise, alcohol consumption, and smoking on the day of treatment.
Q:Will the infusion be painful?
A:The sensation is comparable to a standard infusion, and significant pain is generally not experienced.
Q:Can anyone receive this treatment?
A:This treatment is not suitable for individuals who are pregnant, have severe medical conditions, infectious diseases, or are currently undergoing cancer treatment. Final suitability will be determined by your physician.
Q:How is this different from stem cell therapy?
A:Exosomes are not cells themselves. While the risk of immune rejection is lower, their regenerative capacity is more limited compared to stem cell therapy.
Q:What type of exosomes are used?
A:We use dental pulp-derived exosomes from Japanese donors aged 5 to 19 years.

Medical Reviewer:Dr. Koichi Yoshimi
He has served as the director of major aesthetic surgery clinics, including Otsuka Aesthetic Plastic Surgery, and has long been at the forefront of Japan’s aesthetic medicine field.
He is a member of the Japan Society of Plastic and Reconstructive Surgery, the Japanese Dermatological Association, and the Japan Society for Laser Surgery and Medicine, as well as the Japan Society for Regenerative Medicine and the Japanese Society for Extracellular Vesicles.